No.
First off, you're very close to the accuracy limit of a pH device so a 0.1 drop is barely noticeable.
Second, a pH reading indicates a value at a certain point in time. We're looking for
trends, that's where journaling a grow comes in handy. I used to be an Excel jock so that's what I use for a journal.
Third, dropping pH can be a signal that you've got "organic matter" in the res but let's just refer to it as "root rot" which you will see as a brown, slimy brown discoloration of roots, and it may (will?) have a nasty smell to it. In DWC, it's common for nutrient solution to stain roots and characteristic of that is that the oldest roots will be the darkest (makes sense since the older roots have been in the chemical bath longer than the new roots) but root rot will tend to have a more consistent color vs the graduated change in color of staining from nutes.
Re pH - 5.8 is considered "optimal" but allowing pH to "swing" is a common practice because different chemicals are taken up at different pH values. My practice has been to allow pH to range up to 6.1, mostly 6.0, and then drop it to 5.8.
Dropping pH
can be a symptom of a problem but it is absolutely not a 100% indicator that there
is a problem. When I started growing (early 2021), I followed a chart that's floating around and wasted hours of my life dropping EC to try to get pH to rise. The chart is, generally speaking, accurate but that's sorta like "
Almost all the bullets missed."

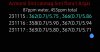
I found it handy to track Water Level, EC, and pH using S(static), F(falling), or R(rising). So falling water, static EC, and static pH would be "FSS". That's a good way to capture the health of the res at a given time and comments in the "Solution" column are good except that
it's common for pH to drop when you replace your res in flower.
How does that happen?
The 17/18 chemicals in "nutrients" are taken up at different rates, per the attached PDF. One of the chemicals that's taken up the most rapidly is K. Per the document, K is in the "fast uptake" group meaning that it's taken up in a few days. When K is removed from a nutrient solution, the pH of that solution will drop.
That's the chemistry side of it. What happens IRL?
A grower on another site, who mixes his own ferts, told me that it was the K uptake but that didn't click until I found the Bugbee paper (attached). The behavior that I've seen mirrors what Bugbee describes exactly (I'm sure Dr. B is relieved to hear that!

) - for the first few days after a new res in flower, pH will drop "quickly" meaning 0.1 units every few hours.
Newbie-me spent hours replacing nute solution with water to drop the EC in my res, trying to get pH to stabilize. IIRC, EC was <= 0.6 before I called bullshit and went back to a normal (1.6 EC) strength res.
And why does that K get inhaled so quickly? Per Bugbee, cannabis takes up K in flower so that it can be stored in the seeds for when they germinate. "But, but, but…", says the astute reader, "we're growing just flowers, no need for the extra K". Bingo. That's why some growers, myself included, run the same nutes from seedling to chop but that's a discussion for another thread.