You can decide where you want your pH. you want to be in the sweet spot without chasing pH.
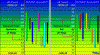 as you can see from the chart on the right if your at 6.1 you have locked out P, Ca and Mg. not the best for your ladies. Your sweet spot is shown so as long as your in the range leave it alone.
as you can see from the chart on the right if your at 6.1 you have locked out P, Ca and Mg. not the best for your ladies. Your sweet spot is shown so as long as your in the range leave it alone.
Here is some more info on pH I took the pix out
^^UNIVERSITY OF
if FLORIDA
IFAS EXTENSION
Managing the pH of container media.
Paul Fisher, Associate Professor and Extension Specialist, University of Florida,
[email protected], Tel 352 392 1831 ext 375, Fax 352 392 3870. April 2 2007. Originally published by University of New Hampshire Cooperative Extension.
Table of Contents:
1. Why is pH important?........................................................................................................................... 1
2. Recognizing the problem .... 2
3. Why do pH problems arise?.................................................................................................................. 2
4. Correcting pH problems........................................................................................................................ 5
4A. Correcting low medium-pH....................................................................................................... 5
4B. Correcting high medium-pH....................................................................................................... 6
Figures. 8
1. Why is pH important?
The most common nutritional problems occur in greenhouse crops when pH of the growing medium is outside the optimum range. Medium-pH is a measure of the acidity (low pH = acid) or basicity (high pH = basic, also called alkaline) of the growing medium. The pH of a growing medium is important because it affects a chain of events:
pH determines micronutrient solubility in the root medium:
low pH = very soluble
high pH = less soluble
Plant takes up soluble nutrients through roots
Nutrients transported into
leaves and growing points:
Excess = toxicity
Adequate = healthy
Insufficient = deficiency
Toxicity or Deficiency results in stunted growth and poor plant appearance
Plants only take up dissolved nutrients through their roots. Medium-pH drives the chemical reactions that determine whether nutrients are either available for root uptake (i.e. soluble) or unavailable for uptake (insoluble). Several nutrients are affected by medium-pH, but the most important are phosphorus and most micronutrients, especially iron, manganese, copper, zinc, and boron (which decrease in solubility at high pH), and molybdenum (which increases in solubility at high pH).
The optimum range for most crops growing in a soilless medium is 5.8 to 6.4, because in this range micronutrients are soluble enough to satisfy plant needs (Figure 1) without becoming so soluble as to be toxic.
Figure 1. The effect of growing petunia at different medium-pH's on (A) Media iron content (from a saturated media extract using deionized water as the extractant), (B) Iron content in the tissue, and (C) chlorophyll content. This figure shows that as pH increased there was a decrease in the available iron in the medium, and less uptake of iron into the leaves. At the highest pH, plants showed chlorosis (lack of chlorophyll) because of iron deficiency. Research by Brandon Smith and Paul Fisher, Univ. of New Hampshire, and William Argo, Blackmore Co.
2. Recognizing the problem
At high medium-pH, micronutrients (especially iron) become less soluble in the medium, and deficient in plant tissue. Iron is required to produce chlorophyll (the green pigment in leaves). If iron is insoluble in the medium because of high medium-pH, then new leaf tissue grows faster than roots can supply the necessary iron. Symptoms of the resulting iron deficiency (Figure 2) are chlorosis (yellowing from lack of chlorophyll) in the younger foliage, which is sometimes interveinal (between leaf veins). As deficiency becomes more severe, foliage becomes almost completely white and necrotic (dead) areas form at the growing points.
At low medium-pH, iron and manganese are highly soluble in the medium. Excess micronutrients can accumulate in plant tissue, and cause chlorosis and necrosis (dead tissue) on leaf margins and as leaf spots (Figure 3). The damage tends to occur in older leaves because the longer a leaf grows on the plant, the more time it has to accumulate excess micronutrients.
3. Why do medium-pH problems arise?
Some of the reasons medium-pH can be too high or low include:
1. Poor buffering of soilless media. In the last 20 years, the move away from use of soil in greenhouse container media has resulted in less buffering (chemical resistance to pH change). In peat and bark-based media, a change of up to 1 pH unit in a week can sometimes occur in commercial crops. Although use of soilless media has many benefits (uniformity, consistency, aeration, sterility) one downside is that pH is very likely to change over time even if the medium starts out at the optimum pH range at time of planting. pH can drift up or down depending on the balance of factors including water alkalinity, lime activity, acidification of the medium by plant roots, and use of an acid or basic reaction fertilizer. As a result, it is important not just to blame problems on the media, but rather to also understand how grower management can cause pH to change over time.
2. Lime. Lime is mixed into media to raise pH to around 6.0, because both peat and bark are acidic. Limes differ in their source and particle size, which causes them to vary in how reactive they are (i.e. how many lb/cubic yard are required to raise pH at the start of the crop), and also in how long they continue to react during crop growth. If the incorrect type or quantity of lime is used during mixing of the medium, pH can either be out of range at the start of the crop, or drift over time. If mixing your own medium, (a) consult a fertilizer or media company to obtain a suitable type of lime, (b) run small batch tests to check how much lime is needed to bring pH up to the target level, and (c) if you change the source of lime, peat, bark, or vermiculite you will need to re-test your recipe. If you consistently run into problems with high or low medium-pH, and you have correctly matched the fertilizer type with water alkalinity, consider changing the lime type or rate.
3. Wide range in crops. Species differ in their nutritional needs, and can be separated into three nutritional groups based on their efficiency at taking up micronutrients (Figure 4):
Petunia group (iron-inefficient, prone to iron deficiency at high pH, especially combined with low fertilizer concentration). Examples include calibrachoa, diascia, nemesia, pansy, petunia, snapdragon, vinca. Grow at a lower pH range of 5.4-6.2 to increase solubility of micronutrients. This group is often misdiagnosed as a "high feed" group - they do not necessarily require more nitrogen, phosphorus and potassium (N, P, and K) than other crops, but they are especially sensitive to high pH and the need for adequate iron.
General group (e.g. chrysanthemum, impatiens, ivy geranium, poinsettia). Grow at a moderate pH range of 5.8-6.4.
Geranium group (iron-efficient, prone to iron/ manganese toxicity at low pH, especially when combined with high fertilizer concentration). e.g. marigold, seed and zonal geranium, New Guinea impatiens, lisianthus. Grow at a higher pH range, 6.0-6.6 to limit the solubility of micronutrients.
Strategies for managing multiple crops include:
Train your staff to recognize the likely problems with each group of plants - micronutrient deficiency for Petunia, and toxicity for Geranium.
For the Petunia group, use a more acidic fertilizer (more ammonium) or less pre-plant lime compared with the Geranium group.
Organize greenhouse zones so that plants within the same nutritional group can be managed with one injector or fertilizer tank.
If using one injector for everything, you may need to apply flowable lime to the Geranium group if medium-pH is below 6.0, or supplement iron for the Petunia group if pH is above 6.3.
Managing media-EC and medium-pH go hand in hand. High fertilizer concentration and high medium-EC increases risk of toxicity for the Geranium group (Figure 5) grown at low pH. In contrast, deficiency symptoms in the Petunia group are more likely with low fertilizer concentration and low medium-EC in combination with high pH.
When growing a range of species (e.g. a mixed basket) maintain pH 6.0 to 6.2.
4. Fertilizer type
You cannot measure the acid or basic reaction of a water-soluble fertilizer using a pH meter in the stock tank. Rather, the tendency of a water-soluble fertilizer to change medium-pH depends on the form of nitrogen used (ammonium, nitrate, or urea). The nitrogen form has a consistent effect on medium-pH:
Ammonium and urea are both acidic (drop medium-pH).
Nitrate is somewhat basic (raises medium-pH).
The acid or basic reaction of a water-soluble fertilizer is written on the bag as an acidic or basic "calcium carbonate equivalency" (CCE), which is a relative measure of the tendency of the fertilizer to raise or lower medium-pH. Examples in Table 1 show that the CCE of acidic and basic fertilizers is affected by how much of the total nitrogen in the fertilizer is contributed by ammonium and urea (the "% Acidic Nitrogen" column in Table 1). "Acidic" fertilizers such as 20-10-20 tend to contain more than 24% of all nitrogen in the ammonium or urea form, and "basic" fertilizers such as 13-2-13 generally have less than 24% of all nitrogen as ammonium or urea.
Several factors are important when using fertilizers to raise or lower medium-pH:
Nitrate only increases medium-pH when the fertilizer is taken up by plant roots. Therefore, if plants are small, or are stressed and not growing, nitrate has little influence on medium-pH.
Ammonium can cause the medium-pH to go down even if the plant is small or is not growing, because soil bacteria acidify the medium through a process termed nitrification.
Ammonium is less effective at lowering medium-pH in cool, saturated soil because nitrification is inhibited. In addition, ammonium toxicity in plants can occur in cool, wet conditions because plants are more likely to take up excess ammonium.
Sometimes ammonium will not drop medium-pH at all, because other factors (especially high lime or water alkalinity) can have a stronger effect on pH than the fertilizer.
5. Managing water alkalinity
Water alkalinity is a measure of basic ions, mainly bicarbonates and carbonates, dissolved in the water. Alkalinity can be thought of as the "liming content" of the water, and irrigating with a high alkalinity water (above 150 ppm CaCO[SUB]3[/SUB] of alkalinity) can cause medium-pH to increase over time. Water alkalinity directly affects change in pH of the growing medium, whereas pH of the water source has little effect on medium-
pH.
Alkalinity can be tested onsite or in a commercial laboratory with a colormetric test kit (paper test strips that are also available are too imprecise for greenhouse needs). Laboratories report alkalinity in a number of different ways including ppm or mg/liter calcium carbonate (CaCO[SUB]3[/SUB]) equivalents of alkalinity (which is used in this section), milliequivalents (meq.) of CaCO[SUB]3[/SUB] alkalinity, and ppm or mg/liter bicarbonate. To convert between units, 50 ppm CaCO[SUB]3[/SUB] alkalinity = 1 meq. CaCO[SUB]3[/SUB] alkalinity = 61 ppm or mg/liter bicarbonate.
Options for alkalinity management are:
Alkalinity can be reduced by injecting acid into the irrigation water. The easiest way to calculate the amount of acid needed is to use acid addition calculator from Purdue University and North Carolina State University which can be downloaded from the internet at the web site
www.ces.ncsu.edu/depts/hort/floriculture/software/alk.html.
It may be feasible to change or blend water sources. Rain water collected in cisterns or ponds and water purified using reverse-osmosis contain little if any alkalinity.
Matching the appropriate fertilizer type to balance alkalinity is the most important decision growers can make to maintain a stable pH (Table 2). A low-alkalinity water should be balanced with a basic (low ammonium) fertilizer. A high-alkalinity water can be balanced with an acidic (high alkalinity) fertilizer, although this is not a good approach in cool, dark weather because of the risk that plants may accumulate toxic levels of ammonium.
6. Regular testing
Basing fertilizer decisions on regular tests (every 1-2 weeks) of medium-pH, medium-EC, and the EC of the fertilizer solution solves 90% of nutritional problems by alerting growers to problem trends before plants are stressed. A soil test of pH is also an easy way to confirm a suspected medium-pH problem. Monitoring other factors (e.g. root diseases, greenhouse temperatures, pest problems, high or low medium-EC) help rule
out these problems, because many factors other than medium-pH can cause the symptoms shown in Figures
2 and 3.
To interpret results from a test of medium-pH:
Low pH problem: Medium-pH is below 6.0 for a seed or zonal geranium crop, marigolds, lisianthus, or New Guinea impatiens (these are crops that tend to accumulate excess iron and manganese at low pH), or below 5.4 for most other species.
High pH problem: Medium-pH is above 6.3 for a petunia, calibrachoa, diascia, nemesia, pansy, petunia, snapdragon, or vinca crop (and other species that tend to be inefficient at taking up iron at high pH), or above 6.6 for most other species.
4. Correcting medium-pH Problems
The following recommendations for raising or lowering medium-pH are intended for crops already under severe stress. Prevention of pH problems is better than relying on a cure, and these actions are intended for crops that would be unsaleable without intervention. Phytotoxicity or staining is very likely with these chemicals, and applications should be tested on a small number of plants before applying to the entire crop. Necrotic tissue will not recover, and the goal is to produce new healthy foliage that will cover damage.
4A. Correcting low medium-pH
When pH falls below the optimum range, the first steps are to (a) stop acidifying water if acid is being injected, and (b) shift to a nitrate-based fertilizer (e.g. 13-2-13 or 15-5-15). Further action is needed if pH has not risen within a week and plants are becoming stressed, especially for a species in the Geranium group when pH is below 6.0, or other crops below pH 5.4. Consider soil drenches with either flowable lime or potassium bicarbonate. Other options (hydrated lime or potassium hydroxide) have specialist uses but are less reliable as a corrective liming material.
Several factors affect the choice between flowable lime versus potassium bicarbonate. Flowable lime has a more predictable and stable effect on medium-pH, without increasing medium-EC. Potassium bicarbonate is easier to apply, however (Figure 4), and should be used on flood floors or when applied through low-volume drippers. Both liming materials are fast-acting and show most of their effect on medium-pH within one day. Following a drench, you can reapply after five days if pH is not up to the optimum range.
To minimize phytotoxicity from flowable lime or potassium bicarbonate, apply in cool weather so the material does not dry quickly on foliage; avoid splashing of foliage during application; immediately rinse foliage with a fine spray; and apply with generous leaching to maximize the effect at low concentration.
Other tips for applying flowable lime:
Apply at 4 qts./100 gallons (10 mL/Liter = 1:100).
An injector can be used to dilute the solution, but the lime particles can be very abrasive. Immediately clean equipment after application.
Do not apply through drippers or on flood floors because it will clog equipment and leave residue.
Other tips for applying potassium bicarbonate:
Apply at 2 lbs./100 gals (2.4 grams/Liter).
Can be delivered through emitters or on flood floors.
One day after application, apply a basic fertilizer (e.g. 13-2-13) with moderate leaching to wash out salts and to reestablish nutrient balance.
It is likely that repeat applications may be needed.
4B. Correcting high medium-pH
Several actions may be necessary when medium-pH is too high.
1. Use a high-ammonium fertilizer combined with low alkalinity. Check with your fertilizer manufacturer to select a high-ammonium (very acidic) fertilizer (e.g. 9-45-15 or 21-7-7). The effect on medium-pH can sometimes be slow (> 1-2 weeks) especially in cool wet conditions, or with small plants growing in large containers. Repeated applications of ammonium in cool, dark conditions may also cause toxic levels of ammonium to accumulate in leaf tissue.
If you have the necessary equipment, and alkalinity is >80 ppm, acidify water to drop the irrigation water-pH to around pH 4.5 (which gives near-zero alkalinity). Continue until medium-pH is in the target range. For the appropriate acid rate for your water source, see
www.ces.ncsu.edu/depts/hort/floriculture/software/alk.html
As a guideline for the commonly-used 35% sulfuric acid ("battery acid") or other acid forms, use the following table as a guideline to drop the pH of irrigation water to 4.5 with different starting alkalinities:
2. Correct micronutrient deficiencies. Masking the symptoms of high pH with micronutrient applications can be very effective for keeping plants alive and healthy when grown under high media-pH conditions. However, unless your customers continue the iron sprays or drenches, or transplant the plants soon after receiving them, quality will suffer. Always use a tissue analysis to test which nutrient is deficient. Although iron deficiency is most common, if a different nutrient (e.g. manganese) is limiting then application of iron may worsen the problem because of antagonistic effects.
Iron comes in different forms that vary in solubility at high pH. Best to worst in terms of effectiveness as a drench at high pH are: Iron-EDDHA "Sequestrene 138" or "Sprint 138" > Iron-DTPA "Sprint 330" > Iron-EDTA > Iron sulfate.
The recommended application rate for an iron drench is 5 oz/100 gal of either Iron-EDDHA (provides 22.5 ppm iron), or Iron-DTPA (37.5 ppm iron). Do not apply to plants in the Geranium group because iron toxicity is likely. Always test on a small group of crops first and check for phytotoxicity after 7 days. The solutions should be applied with generous leaching, followed immediately by washing of foliage to avoid leaf spotting. All options are low cost, at less than 0.1 cents per 4-inch-diameter pot. Iron-DTPA (Sprint 330) can be purchased from greenhouse and nursery suppliers. Ask for Iron-EDDHA (Sequestrene 138 or Sprint 13

from a fertilizer representative.
Foliar sprays are also somewhat effective, especially if iron chlorosis is mild. Suggested iron forms and rates for iron sprays are Iron-EDTA (60 ppm iron, equals 6.1 oz/100 gal) or Iron-DTPA at 60 ppm iron (8 oz/100 gal). Repeat applications are likely to be needed every 5 days because the iron is not transported to new leaves, and the plant can grow out of a foliar spray. Phytotoxicity is likely, and after applying foliar sprays to a test group wait 3 days to check for damage before applying to the entire crop.
Spray application method is very important. Tips for maximum effectiveness of foliar sprays:
Include an organosilicone surfactant (e.g. Capsil[SUP]TM[/SUP] at 13 oz/100 gal)
Apply in early morning on cool, cloudy days for gradual drying of leaves in order to increase uptake and reduce spotting (Figure 9)
Spray both sides of leaves because penetration may be better on the underside of leaves where the cuticle is thinner.
3. Consider acid drenches in extreme cases. Iron sulfate or sulfuric acid drenches can reduce medium-pH but phytotoxicity is very likely. To minimize phytotoxicity with either chemical, apply during a cool morning. Avoid contact with foliage, and immediately rinse foliage after application. Applying with generous leaching.
Aluminum sulfate should only ever be used to drop medium-pH for hydrangeas, because otherwise it is will cause nutrient imbalances. Flowable or elemental sulfur is sometimes used to drop pH in the nursery trade, but tends to cause a gradual reduction in medium-pH over time that is difficult to control (because microbial action is needed for the sulfur to be effective).
Iron sulfate provides iron (which is usually deficient in plants at high pH) in addition to causing a temporary drop in medium-pH. This material increases EC (1.2 dS/m at 2 lb/100 gal) and the excess iron (2 lb/100 gal provides 500 ppm iron) may cause imbalances if pH falls below 6.0. Iron sulfate should NEVER be used as a drench on iron-efficient species (Geranium group) or long-term crops. 2 lb./100 gal is the maximum recommended rate - higher rates up to 6 lbs/100 gal will cause a greater drop in medium-pH, but also have increased risk of phytotoxicity.
Other tips to applying iron sulfate:
Store dark and dry. Iron sulfate oxidizes over time, and has a 6-12 month shelf life. Mix in water with a pH below 7.0 and only use if the final solution is not cloudy.
Can stain media and plastic subirrigation benches black.
Leach heavily with a complete fertilizer after one week to try to remove excess iron and restore nutrient balance.
Sulfuric acid can be injected into water to bring the drench solution pH to 1.5-2.0. Repeated applications are necessary, however, and media vary in how many applications are needed depending on how much they resist pH change. Unlike iron sulfate, sulfuric acid does not leave excess iron in the media that can cause problems later. Sulfuric acid (available as battery acid) is highly caustic to people and plants.
Generally the best approach is to just add enough acid to neutralize alkalinity at pH 4.5, which is a moderate rate that should be combined with continued use of an ammonium-based fertilizer until medium-pH drops. Use Table 3 above, or the web site
www.ces.ncsu.edu/depts/hort/floriculture/software/alk.html) to calculate acid rates.
Other tips to apply sulfuric acid drenches:
Solution pH of 2.0 or lower is very toxic to plants and people. Use equipment that is designed to handle strong acids and use protective clothing. This should only be used in consultation with a fertilizer expert.
Do not mix in fertilizer stock tank without consulting fertilizer manufacturer or precipitation is likely.
Always add acid to water, and not water to acid.
Prevention Is Better Than A Cure
As a final note, remember that medium-pH problems should never cause crop losses if you
1) Set up a sensible nutrient management program that is suited to your crop types and water source,
2) Establish a regular monitoring regime, and
3) Develop strategies that will keep pH and EC on track before you need extreme "rescue" measures.
Acknowledgements: The author thanks the Floriculture Industry Research and Scholarship Trust (FIRST), the UNH Agricultural Experiment Station, and the Anna and Raymond Tuttle Environmental Horticulture Fund for supporting this research. Dr. William Argo, Linda Bilodeau, Jeremy Bishko, Brandon Smith, and Ron Wik contributed to the research presented in this section.


