Daub Marley
Active Member
more specifically its called salting out. Skunk pharm has the details in the polishing section. You're going to need a good non-polar solvent.
Alcohol in general, whether it is 91% ISO, 99% ISO, or even 190 proof Everclear, they are all polar. A non polar solvent would be like butane and propane and such.is 100% iso alcohol polar or non-polar? what is the difference?
ok ive seen a couple threads that show how to spray the butane through a metal turkey baseter. i needed some butane for a couple refillable lighters that were empty,i checked around walmart, kmart, and they dont sell butane. what kind of butane would be best for that process and where can i find it?Alcohol in general, whether it is 91% ISO, 99% ISO, or even 190 proof Everclear, they are all polar. A non polar solvent would be like butane and propane and such.
No I haven't but pretty good information there Texas, thanks!https://www.icmag.com/ic/showthread.php?t=275567
you ever check this out WM?
recommended butane from icmag with mirror test results
ok cool ill try to locate some of that, how many cans do you think i would need for an oz of green?You want to get a butane can that is at least 5x filtered. I use power 5 (its a blue and black can). You can get it at some liquor stores here, head shops, or online.
You can do the mirror test to test the butane that you get. You spray it on a mirror for about five seconds and see if there is any residue.
In my opinion, an open dish with a puddle of liquid butane is more dangerous object than a mason jar or thermos filled with liquid butane., the long soak method (what I do) is more dangerous than spray tube just because you literally have a mason jar filled with butane chillin.
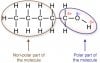
Well it's both. Its a great solvent to clean with because of which. Polarity is when an atom of a molecule has a tug of war with another atom, but instead of pulling straight the opposite direction (non-polar) they pull in somewhat the same direction and the forces do not completely cancel each other out. This make the molecule become polar, sort of like a magnet. The negative end of a polar molecule wants to connect to a positive end of another polar molecule. This can force out any non-polar molecules like with oil(non-polar) and water(polar).is 100% iso alcohol polar or non-polar? what is the difference?
well see this all stems from what i did with some stuff that i grew that wasnt very good, i tried a water cure on it, soaked it for a week and got all the chlorophyll and crap out of it, then dried it. the taste was better but a bit bland, like some good reggie but still had major trichs on it. i figured that the taste would suffer but a cleaner final product is what im after. and im probably going to stick with qwISO for now, until i get the hang of it. i just want to get to full melt, so it doesnt keep caking up and burning out my vape coil.I think that putting your material in water for a week first will help the final product, except for taste. Potency will be slightly better. Look up "water curing". I didn't believe it at first but it works great for me. You will lose anywhere from 10-30% (closer to 10 with nugs) in the original weight of your product, but your original problem of having the extra "plant" materials will be solved. I was actually wondering and considering doing this same thing.
To find out the "polarity" of a solvent, you can check the dielectric constant. Any thing with a dielectric constant above 15 is considered "polar", with water, the universal solvent, at 80. Check out the Epsilon column on the graph at the following site: http://depts.washington.edu/eooptic/linkfiles/dielectric_chart[1].pdfWell it's both. Its a great solvent to clean with because of which. Polarity is when an atom of a molecule has a tug of war with another atom, but instead of pulling straight the opposite direction (non-polar) they pull in somewhat the same direction and the forces do not completely cancel each other out. This make the molecule become polar, sort of like a magnet. The negative end of a polar molecule wants to connect to a positive end of another polar molecule. This can force out any non-polar molecules like with oil(non-polar) and water(polar).
View attachment 3020945
