Guile
Active Member
Im sorry, I was drunk earlier and (was being a smart ass) added instead of subtracted which led to the wrong temperature.
Anyways someone (like me) will put dry ice in a metal container that is not nearly strong enough and heat it up a little resulting in an explosion.
My explanation for Co2 canisters not exploding in pockets?
They are only like 37% liquid and the rest gas, also made of high grade aluminum,
Not only that but they certain rupture points to vent in the case of high temps increasing pressure to much.
If you put dry ice into a less then ideal container, which someone might try, it will explode.
Only thing is I've done it on purpose, and if someone were next to that they would have been blown to pieces.
Sorry for being a dick.
Thanks man... Hope you know my rudeness was strictly superficial, I hate being an ass (but you have to stand up for yourself/others once in a while, otherwise you come off as a pushover).
I looked into the working and burst pressures of the pipes I was speculating about using, they are within technically safe limits for the pressures we would be working with using Co2.
Keep in mind that we are talking about using dry ice "nuggets" (as they are sold locally to me) so it would be nearly impossible to fill the entire volume of your pipe with solid Co2 (at the most 75% assuming they were perfect identical spheres) .. Its kind of like filling a pipe with marbles, there will be plenty of gas space (voids) left inside (like your Co2 cartridge). Besides I never advocated filling the pipe more than 1/2-2/3 full of dry ice nuggets as the density of unconfined dry ice is about 2-3 times that of liquid at the temperatures and pressures we have discussed, meaning the liquid it should take up around twice the volume @ 1100psi and 90F if I'm not mistaken. As long as your vessel is less than half fill of solid Co2 (or 3/4 full of nuggets) you should have sufficient space to avoid pressure issues as the solid converts to a gas.
I have advocated using the "burst disks" from Co2 tanks for safety purposes, they have a failure point between 2500-3000psi which is high but only around twice the working pressure and half the burst pressure of the pipe I suggested using to make your vessel...
I'm not advocating to anyone to do anything on blind faith in my word.. Only offering the information needed to begin their own independent research/development.. Always double check the specifications of your components (then triple check)..
I think the biggest concern would be that the unconfined density of dry ice is around 1.562 g/mL.
At the critical point of Co2: 31.3C (88.34F) @ 72.8 bar (1056psi) density is 0.467g/ml (about 1/3 as much as dry ice).
As pressure increases so does density however as temperature increases density lowers (making volume and temperature key considerations)
If you don't account for the space necessary to accommodate the volume of liquid Co2 (at temperature) your pressures could rise exponentially leading to containment failure.
Hope you didn't wake up with a hangover, and that you are having a good weekend...
-------------------------------------------------------------------------------------------
looks like at around 1300psi & 100c (212F) Co2 will have a density around 0.2g/ml
At around the same pressures but 32c (90F) Co2 will have a density around 0.7g/ml
looks like at around 1100psi & 100c (212F)Co2 density will be around 0.15g/ml
At around the same pressures but 32c (90F) Co2 will have a density around 0.6g/ml
Using those figures as guides you can figure out how much dry ice is safe to put into your pressure vessel..
For instance if you used a 4" sch 40 pipe 6 foot long it would have a volume of around 900 cubic inches (about 14750 cubic centimeters). If you wanted to heat it to 100C @ 1100psi you could only fit in 0.15 grams dry ice per cc/ml giving you about 2,200 grams (about 4.75lbs) of dry ice..
However, using the same chamber If you wanted to heat it to 32C @ 1300psi you could only fit in 0.7 grams dry ice per cc/ml giving you 10,000 grams (about 22lbs) of dry ice. This is in the ballpark of my last idea before considering phase change systems, however one could be built around this vessel as a condenser/high pressure reservoir. Obviously you would need a compressor and evaporator/catch container for your extracts (the rest is just pluming, gauges and valves).
By the way, brake lines and fittings are made to handle these kinds of pressures (double flare everything for good measure).
These are all estimates and best guesses... I would still recommend doing more research and adding Co2 tank burst disks, not to mention every bit of care/caution you can muster if you plan to try this.

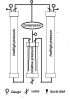
The second drawing depicts a setup that allows you to continually recycle your Co2 by diverting it into a second extraction chamber while refilling the first (such a system would not need the addition of dry ice with every batch of trim)...


